In the field of neoantigen therapy, NeoCura has built a unique Drug Screening & Validation platform, which, at molecular and cellular levels, can accurately and rapidly identify the neoantigen combination(s) with the best effect from a variety of predicted neoantigens. Based on this platform, an efficacy assessment system is established accordingly to accurately assess the clinical effect of the neoantigen therapy in patients.
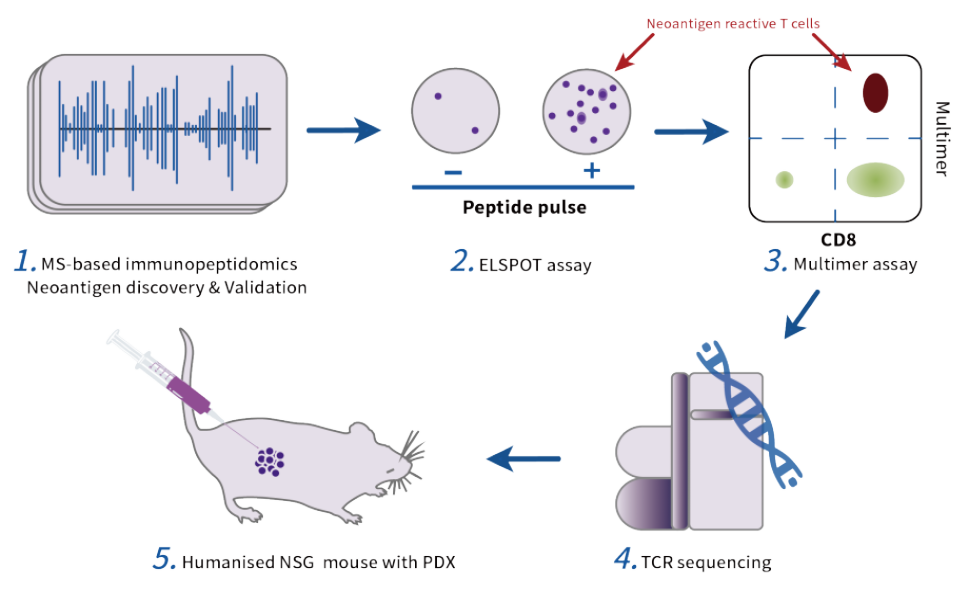
Technical superiority

Employing proprietary attomole-level (10 mol) high-precision mass-spectrometric analysis platform (LC/MS/MS) to detect the HLA binding peptides on tumor cell surface directly;






